Transcranial magnetic stimulation is a non-invasive and non-systemic treatment that stimulates a small region of the brain back into health.
It is a safe therapeutic modality that has been used in medicine since 1980s but only recently received FDA clearance to treat depression. The magnetic energy that the brain receives during the entire 20-30 sessions of TMS is a small portion of what patients are exposed to during a single brain MRI.
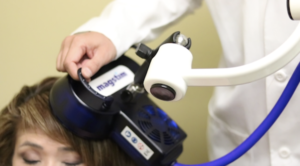
How does Brain TMS Therapy work?
TMS Therapy utilizes an electromagnetic pulse to stimulate regions of the brain that are suppressed in patients with depression. This raises neurotransmitter levels in these parts of the brain and reduces symptoms of depression.
How do magnets help?
Transcranial Magentic Stimulation (TMS), uses an electromagnetic coil treating only a localized region of the brain. TMS magnetic fields only affect a localized region of the brain since the magnetic pulses reach only about 2-3 centimeters into the brain directly beneath the treatment coil.
As magnetic fields move into the brain, they produce very small electrical currents. The electrical current creates a magnetic field, which passes into the brain and causes neurons to fire electrical impulses. The electrical impulses encourage a chemical reaction (release of neurotransmitters) that, over time, helps reset the brain connections to heal mind and body.
Who can benefit from Brain TMS Therapy?
TMS is the ideal treatment for patients suffering from medication resistant depression.
Therapy has shown benefit to treat various neurological and psychiatric conditions depending on which region of the brain is stimulated. Some of the conditions being successfully treated with rTMS throughout the world are depression, chronic pain, fibromyalgia, migraines, anxiety disorder, post traumatic stress disorder (PTSD), tinnitus, stroke rehabilitation, addiction, Parkinson’s disease and Alzheimer’s dementia.
How often are treatments?
Patients are treated for 20-40 minutes, 5 days/week for 4-6 weeks with additional maintenance sessions as needed.
NOTICE TO PATIENTS OPEN PAYMENTS DATABASE
For informational purposes only, a link to the federal Centers for Medicare and Medicaid Services (CMS) Open Payments web page is provided here. The
federal Physician Payments Sunshine Act requires that detailed information
about payment and other payments of value worth over ten dollars ($10) from
manufacturers of drugs, medical devices, and biologics to physicians and
teaching hospital be made available to the public.
You may search this federal database for payments made to
physicians and teaching hospitals by visiting this website:
https://openpaymentsdata.cms.gov/


